Hf Strong Or Weak Electrolyte
What's the divergence between strong and weak electrolyte?
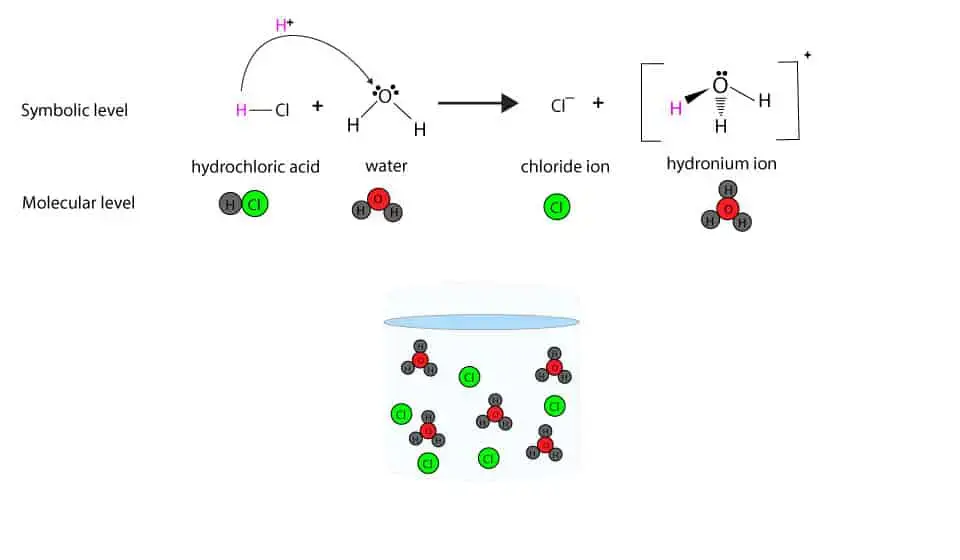
The difference is that a strong electrolyte dissociates completely in h2o, while a weak electrolyte partly dissociates. For case, hydrochloric acid (HCl) and hydrofluoric acrid (HF) dissociate in water to produce H+( these reactive H+ ions share a lone pair of electrons on the oxygen cantlet of water (HtwoO) to course the hydronium ion, H3O+. Therefore, we are more correct if we say that HCl and HF dissociate in water to produce H3O+ )
If you test the conductivity of the same concentration of HCl and HF, you lot volition notice that the low-cal seedling of the conductivity tester glows brighter in the HCl solution than information technology glows in the HF solution. This difference in brightness is considering of how well each chemical dissociates in water.
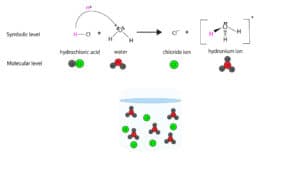
For case, HCl normally dissociates 100% in water. Therefore, HCl is a strong electrolyte.
Here is a model showing how HCl dissociates in water
On the other mitt, only a small fraction of HF molecules dissociates in h2o.
Here is a model showing how HF dissociates in water
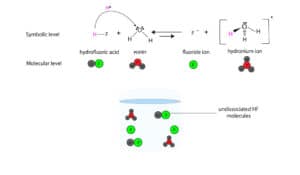
As you can see, nosotros have a longer arrow pointing towards the reactants and a shorter arrow pointing towards the products. The longer arrow ways that the reverse reaction is preferred, and that the products easily combine to become back the reactants. On the other paw, the shorter arrow means that the forwards reaction is non preferred, and that merely a pocket-sized fraction of HF dissociates in h2o.
Therefore, HF is a weak electrolyte.
Potent and weak electrolytes can also be applied to bases and other compounds that dissolve in water to produce ions. Examples of bases that are potent electrolytes include sodium hydroxide and many more than. Examples of bases that are weak include ammonia and many more.
To read more nearly solutions that tin bear an electric electric current, click here.
Hf Strong Or Weak Electrolyte,
Source: https://masterconceptsinchemistry.com/index.php/2017/10/29/whats-difference-strong-weak-electrolyte/
Posted by: shiresiderear.blogspot.com


0 Response to "Hf Strong Or Weak Electrolyte"
Post a Comment